What Is the Value of Temperature and Pressure at Stp
Chemists often measure pressure in kPa kilo Pascals in which atmospheric pressure 100 kPa 1 kPa 1000 Pa. What temperature is absolute zero.

Question Video Identifying The Conditions For Standard Temperature And Pressure Nagwa
What is the volume of 1 mole of a gas at STP.

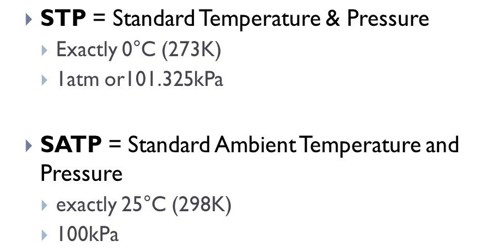
. Certain properties of matter such as. The standard temperature is zero º Celsius which corresponds to 32 F or 27315 K. Standard Temperature and Pressure STP is defined as 0 degrees Celsius and 1 atmosphere of pressure.
STP is an abbreviation for standard temperature and pressure. 25 C 110 atm 9. Standard temperature and pressure STP refers to the internationally agreed-upon standard of measurement for experiments in chemistry.
The standard pressure is 1 bar or 100000 kPa. STP stands for standard temperature and pressure. 48 A P c O 2 M HbO 2 49 B 1 DL CO P L V A.
NIST uses a temperature of 20 C 29315 K 68 F and an absolute pressure of 1. The conditions at STP are. Standard temperature and pressure abbreviated STP refers to nominal conditions in the atmosphere at sea levelStandard temperature is defined as zero degrees Celsius 0 0 C which translates to 32 degrees Fahrenheit 32 0 F or 27315 degrees kelvin 27315 0 K.
Because the standard varies by industry its good practice. According to the International Union of Pure and Applied Chemistry IUPAC the currently accepted values for standard temperature and pressure are 27315 K 0 C and exactly 100kPa 0986923 atm kPa kilopascal. The value that describe temperature and pressure STP is 27315 K and 1013 KPa answer D Explanation International union of pure a.
Since 1982 STP is defined as a temperature of 27315 K 0 C 32 F and an absolute pressure of exactly 10 5 Pa 100 kPa 1 bar. In Chemistry STP is an elementary concept. Since the volume of a gas depends on the temperature and pressure one mole of an ideal gas at STP conditions has a volume of 224 liters.
10 5 Pascals formerly 1 atm but IUPAC has since changed this standard. Pressure and temperature of this system are considered as 760 torr and 273K correspondingly. It is a set of conditions for temperature and pressure measurements for physical and chemical reactions.
All volumes are STPD standard temperature and pressure dry ie. Under these conditions one mole of a gas occupies 224 L. STP is used in many thermodynamic calculations and tabulations.
14 rows Standard Temperature and Pressure defined by IUPAC Standard temperature is 0 or 27315 K or 32. STP should not be confused with the standard state commonly used in thermodynamic evaluations of the Gibbs energy of a reaction. 27315 K 0C or 32F Pressure.
The standard temperature and pressure STP is 0 degrees Celsius and 1 atmosphere of pressure respectively. NIST uses a temperature of 20 C 29315 K 68 F and an absolute. What are the values for standard temperature and pressure.
27315 K 100 atm C. Explain the terms STP and NTP And give their values. Standard Pressure 100 x 10 5 Pascals Nm-2 Standard Temperature 27315 Kelvin.
1 atm and 0C and the various terms in the two forms of the equation are as follows. Do not confuse STP with the STP Products Company a maker of oil and fuel additivesunless you are reading one of their Safety Data Sheets. STP values are most often cited for gases because their characteristics change dramatically with temperature and pressure.
What are the values for standard temperature and pressure STP in units of C and mmHg. Since 1982 STP has been defined as a temperature of 27315 K 0 C 32 F and an absolute pressure of exactly 10 5 Pa 100 kPa 1 bar. 25 С 100 atm b.
DoC and 1 atm. These variable quantities are controlled by agreeing to a worldwide definition of standard temperature and pressure called STP. This volume can be found using the ideal gas law.
You can compute the volume of gas in STP using the standard formula. 0C and 760 mmHg. Because the volumetric value that is associated with this equality is only valid at an experimental temperature of 273 Kelvin and under 1 atmosphere of pressure the phrase at STP must be present to indicate that an STP equality should be used to solve the given problem.
STP should not be confused with the standard state commonly used in thermodynamic evaluations of the Gibbs energy of a reaction. 0C 110 atm d. These standards have been set by the IUPAC.
3 on a question What is the volume at STP standard temperature and pressure of a sample of CO that originally has a volume of 74 mL at 30 C and 680 mm of Hg. STP - Standard Temperature and Pressure - is defined by IUPAC International Union of Pure and Applied Chemistry as air at 0 o C 27315 K 32 o F and 10 5 pascals 1 bar. In the STP system both pressure and temperature are in their typical form.
STP - commonly used in the Imperial and USA system of units - as air at 60 o F 520 o R 156 o C and 14696 psia 1 atm 101325 bara. For STP conditions the temperature is 23715K and pressure is 1 bar. The pressure of a has at STP is 1 bar.
What are the temperature and pressure conditions of Standard Temperature and Pressure STP. In this standard formula the pressure is in torr and temperature is in Kelvin. One common definition of STP is a temperature of 273 K 0 Celsius or 32 Fahrenheit and the standard pressure of 1 atm.
What is a pressure of a gas at STP.

Standard Temperature And Pressure Stp Chemistrygod
No comments for "What Is the Value of Temperature and Pressure at Stp"
Post a Comment